Silicon-containing drugs have attracted a lot of attention in recent years, and two new drugs have been successfully marketed (Akalux: ADC for head and neck cancer; Poslum: Diagnostic reagent for prostate cancer). The introduction of silicon into drugs have shown promising value in improving activity, reducing toxicity and enhancing tissue permeability. Chirality is an important core of drug molecules. Therefore, the inevitable scientific problem in the research and development of silicon-containing drugs is: how to achieve asymmetric synthesis of Si-stereogenic silanes. Compared with the traditional diastereoisomer preparation/separation and the desymmetrization of prochiral silicon, enantioconvergent synthesis with racemic silicon a more efficient strategy strategy, but due to its great challenge, few reports have been reported.
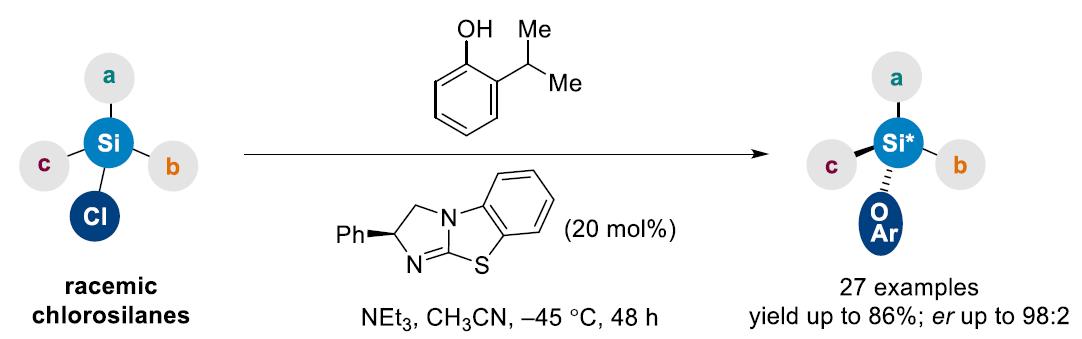
Recently, Professor Song Zhenlei's research group has developed the first chiral Lewis base catalyzed dynamic kinetic asymmetric transformation (DYKAT) of racemic chlorosilane, which provides a new catalytic model for the asymmetric synthesis of Si-stereogenic silanes. Both the catalyst and phenol required for the reaction are commercially available, and have also demonstrated a good range of substrate applications. DFT (density functional theory) calculations revealed that racemic chlorosilane achieves rapid racemization through pentacordinate silicate under the activation of catalyst, thus driving the enantioconvergent synthesis of Si-stereogenic silylethers. (J. Am. Chem. Soc., 2024, 146, 23092-23102, https://doi.org/10.1021/jacs.4c04390).
Address: No. 17, Section 3, Southern Renmin Rd. Chengdu 610041 China
Tel/fax: +86-28-85501628
Email: yxyban@163.com
